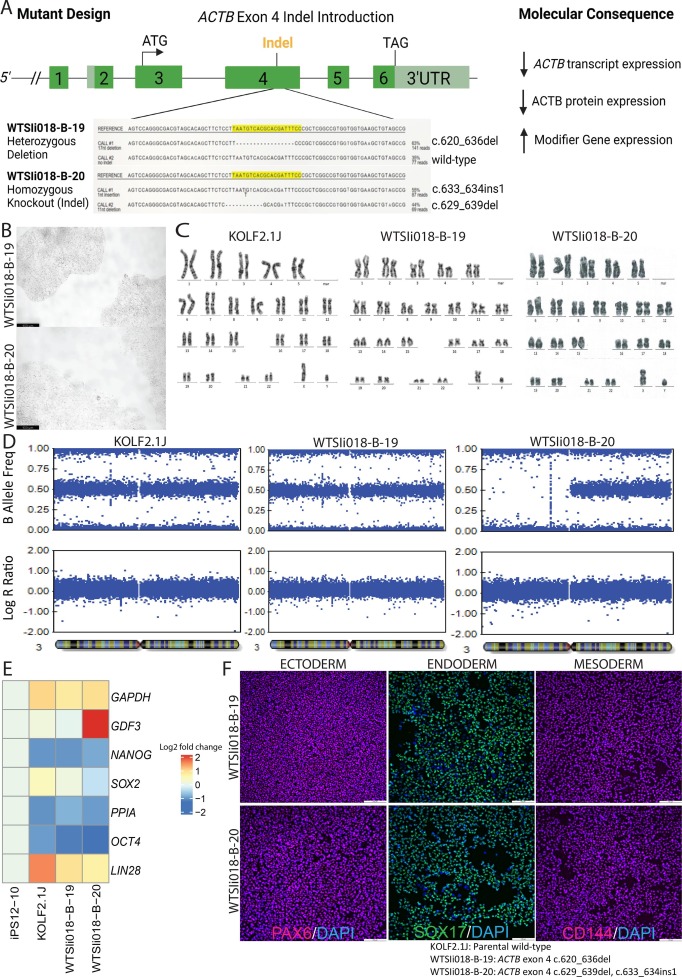
Heterozygous beta-actin (ACTB) indel and nonsense mutations are linked to developmental disorders. We generated two CRISPR/Cas9 human induced pluripotent stem cell (iPSC) lines, WTSIi018-B-19 and WTSIi018-B-20, with heterozygous and homozygous indel mutations in ACTB exon 4. Both lines exhibited normal cell morphology, expression of pluripotency markers, and the ability to differentiate into the three primary germ layers. While heterozygous ACTB mutant iPSCs maintained genome integrity, homozygous mutants showed a loss of heterozygosity in chromosome three. These models are powerful tools for studying the onset, progression, and genetic compensation in ACTB-related diseases.
Mutations in a gene called beta-actin (ACTB) can cause developmental disorders. We used CRISPR/Cas9 technology to create two types of human stem cell lines with mutations in ACTB. These stem cells, named WTSIi018-B-19 and WTSIi018-B-20, had one and two copies of the mutated gene, respectively. Both types of cells looked normal, could turn into any cell type in the body, and showed signs of being fully functional stem cells. However, while cells with one mutated gene were stable, those with two mutated genes lost some genetic material on chromosome three. These stem cell models help us understand how ACTB mutations affect development and how the body might try to compensate for these genetic changes.