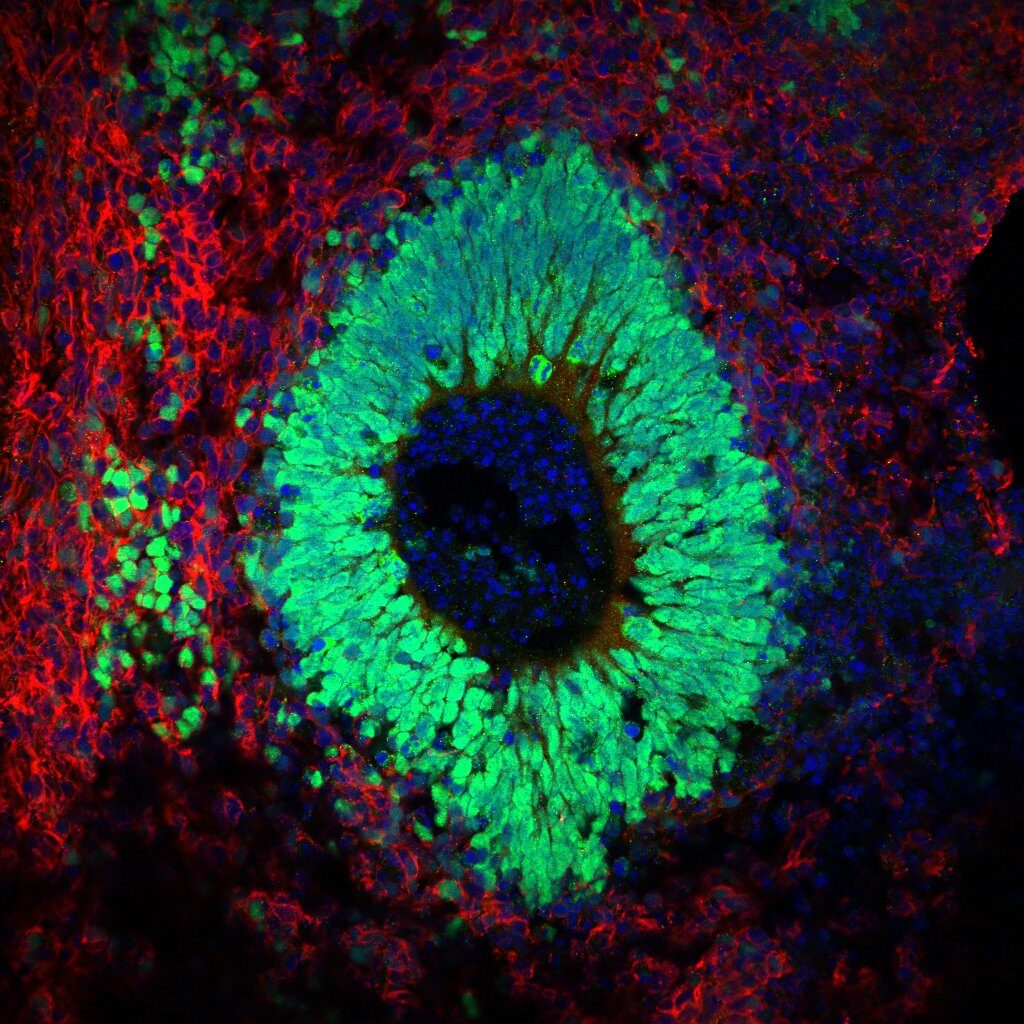
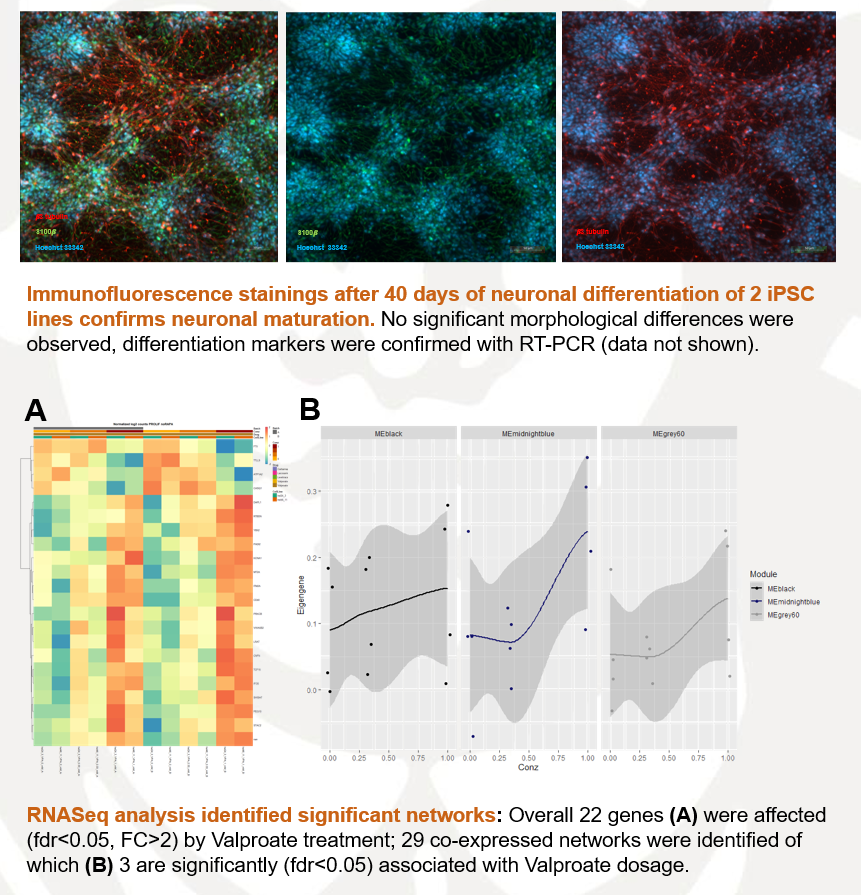
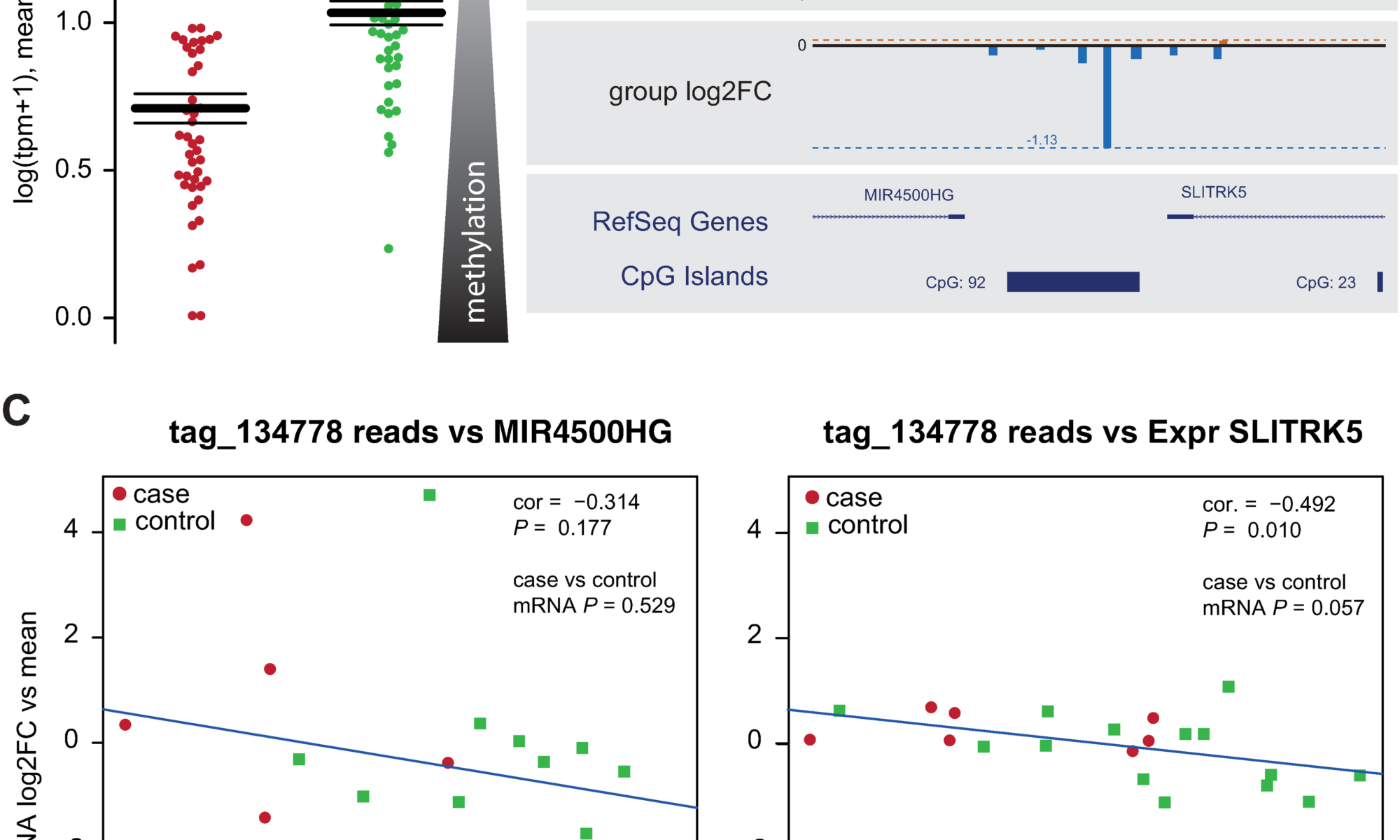
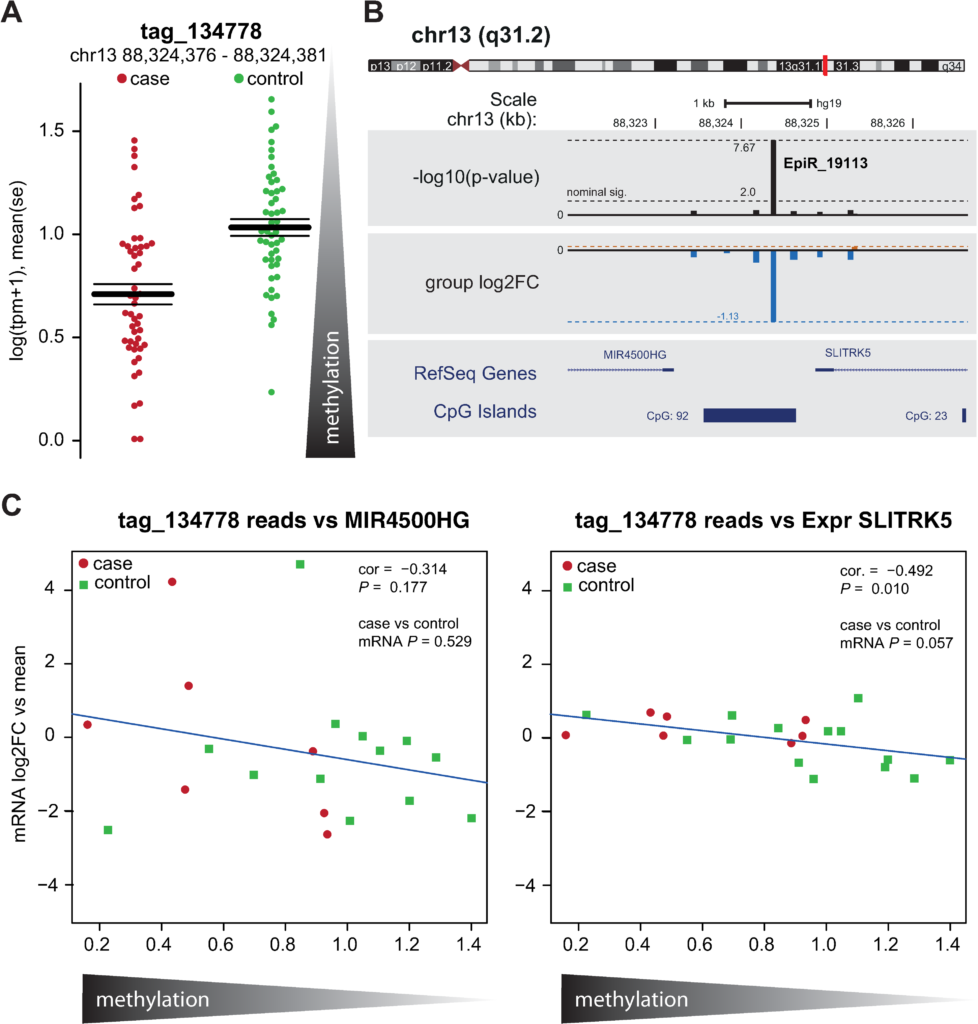
chiocchetti
Experts from 26 countries have laid the foundations in Paris for innovative approaches to improving the quality of life of people with neurodevelopmental disorders. The international and interdisciplinary cooperation, in which our Laboratory and the Clinic for Psychiatry, Psychosomatics and Psychotherapy of Childhood and Adolescence at Frankfurt University Hospital is also involved with three research projects, is being funded by the EU with a total of eleven million euros until 2027.

Embracing developmental diversity as factor for well being
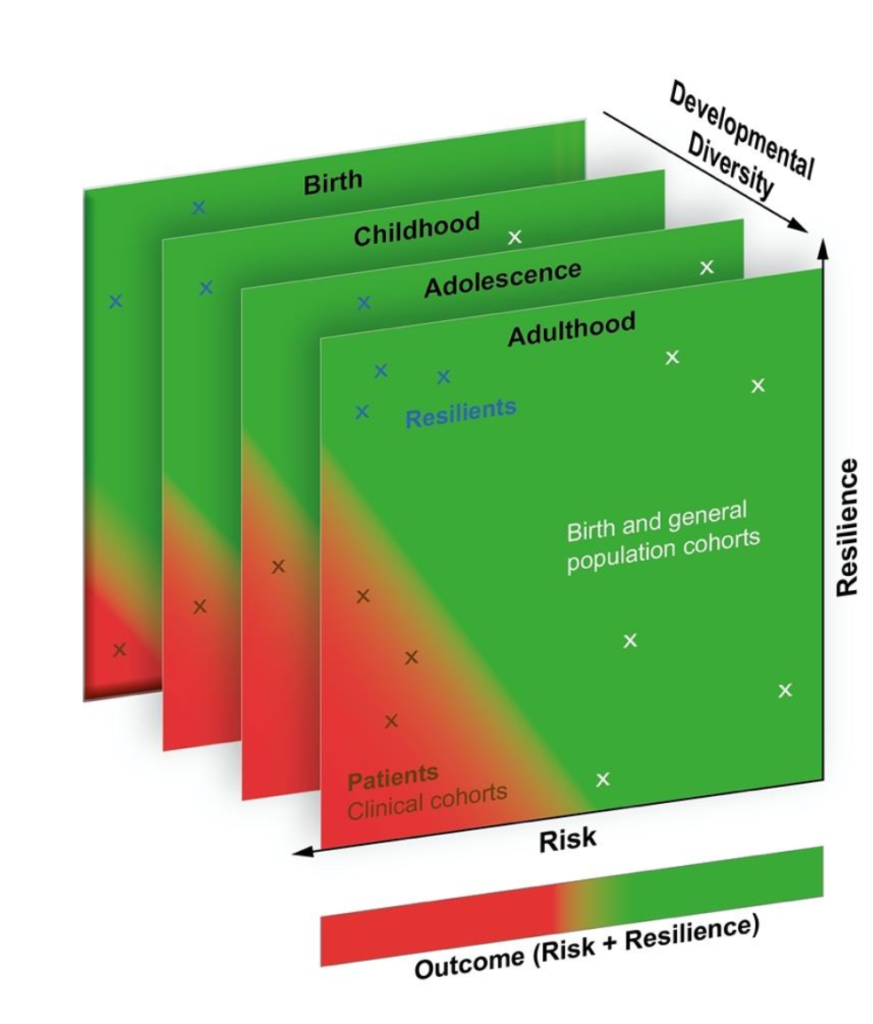
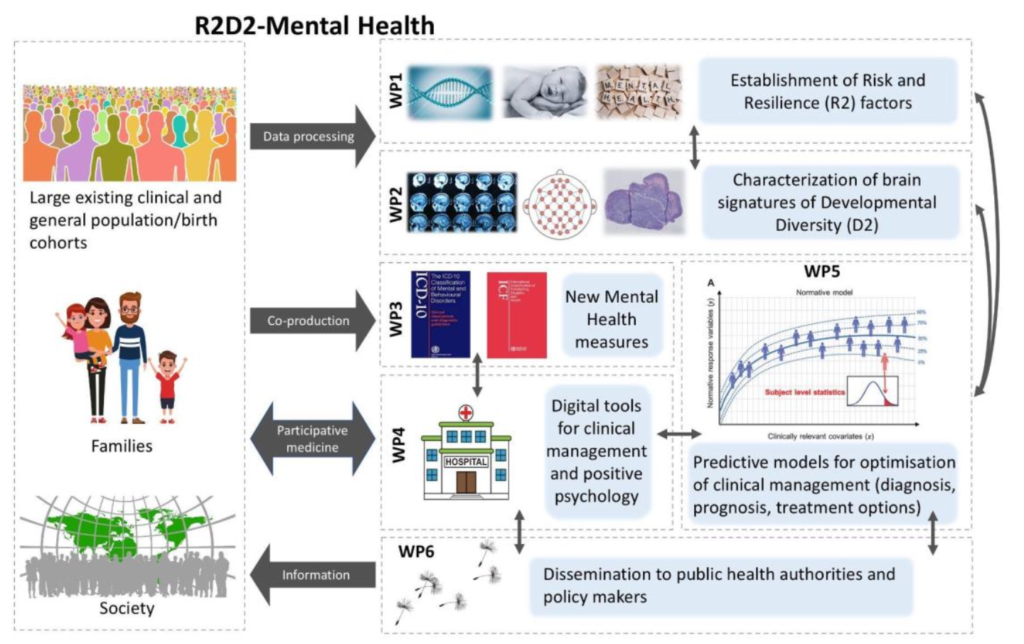
Together with 26 world-leading interdisciplinary research groups from Europe and associated countries, we are committed to improving the quality of life of individuals with neurodevelopmental disorders. The EU-funded project Risk and Resilience in Developmental Diversity and Mental Health (R2D2-MH) adopts a new approach: We are not studying only the risk but we will consider developmental or genetic diversity, as well as a diagnosis and life experiences as factors that influence mental well-being and functioning across the lifespan.
Large proportion of the population affected - neurobiological mechanisms little researched
Mental Health defines personal well-being and 38,2% of the population in the EU population are faced with a mental disorder or challenge which cost EU economies over €600 billion per year. Neurodevelopmental disorders (NDDs) are conditions affecting growth and development in the brain and typically start early in life. These include conditions such as autism, attention deficit / hyperactivity disorder (ADHD). People with NDDs and their families experience greater discrimination and stigma which has a further negative impact on well-being. Currently, there are no highly effective, evidenced based approaches to improve medium to long-term MH outcomes in the context of NDDs. This is due partly to a limited understanding of the neurobiological mechanisms involved in the transition from MH to illness throughout the life course and the interaction with environmental factors. The distinction between diversity and disorder is exactly where R2D2-MH comes in.
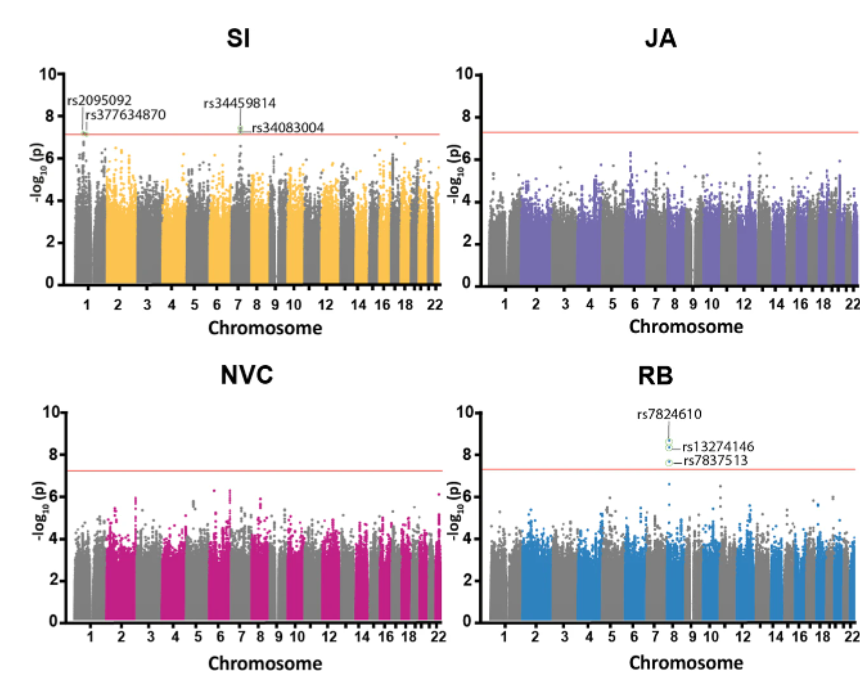
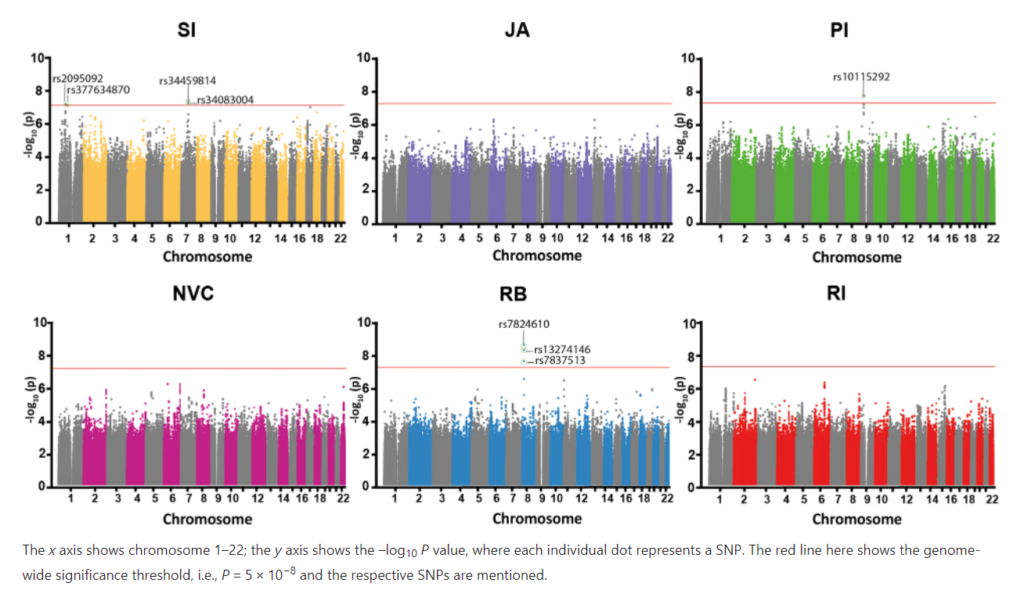
Towards a risk and resilience based personalized intervention
The Department for Child and Adolescent Psychiatry, Psychsosomatics and Psychotherapy of the University Hosptial Frankfurt will be involved in two workpacakges. The reserach group around Professor Ecker will focus on the characterization of risk and resilience markers of developmental diversity using brain imaging data in WP2 and combine them with gene expression, genetic as well as cell model data. Our laboratory will investigate in collaboration with Prof. Freitag in WP4, how treatment interventions interact with genetic markers on the outcome in neurodevelopmental disorders.
Inherent to all WPs is the co-creation with individuals that experience mental health problems in order to optimize study design and the defined outcome measures to ultimately increase the quality of life and well-being.
I am very excited by the idea to co-create the aims of our research with individuals that experience mental health challenges in their every day life.
By combining machine learning and genetics with clinical data-science and a patient oriented outcome definition, we will understand better who will benefit from which available treatment.

With the work in R2D2, we aim at understanding how different types of early intervention are modulated by genetic factors. Thus, we will be able to better understand why some individuals benefit from treatment and others don't.
For this we will collaborate with partners from Geneva (CH) and Queensland (AU) to compare cohorts with different background and treaments.

Deepening our understangin on how the the brain develops and how the genetics of neurodevelopmental phenotypes like Autism Spectrum Disorder are connected to it, will provide the most promising approach to disentangle the effec of neuronal diversity on peronal well being.
In R2D2 we are thrilled to work together with clinical and basic research partners in order to understand how neuronal markers are realted to mental well-being.

Previous
Next